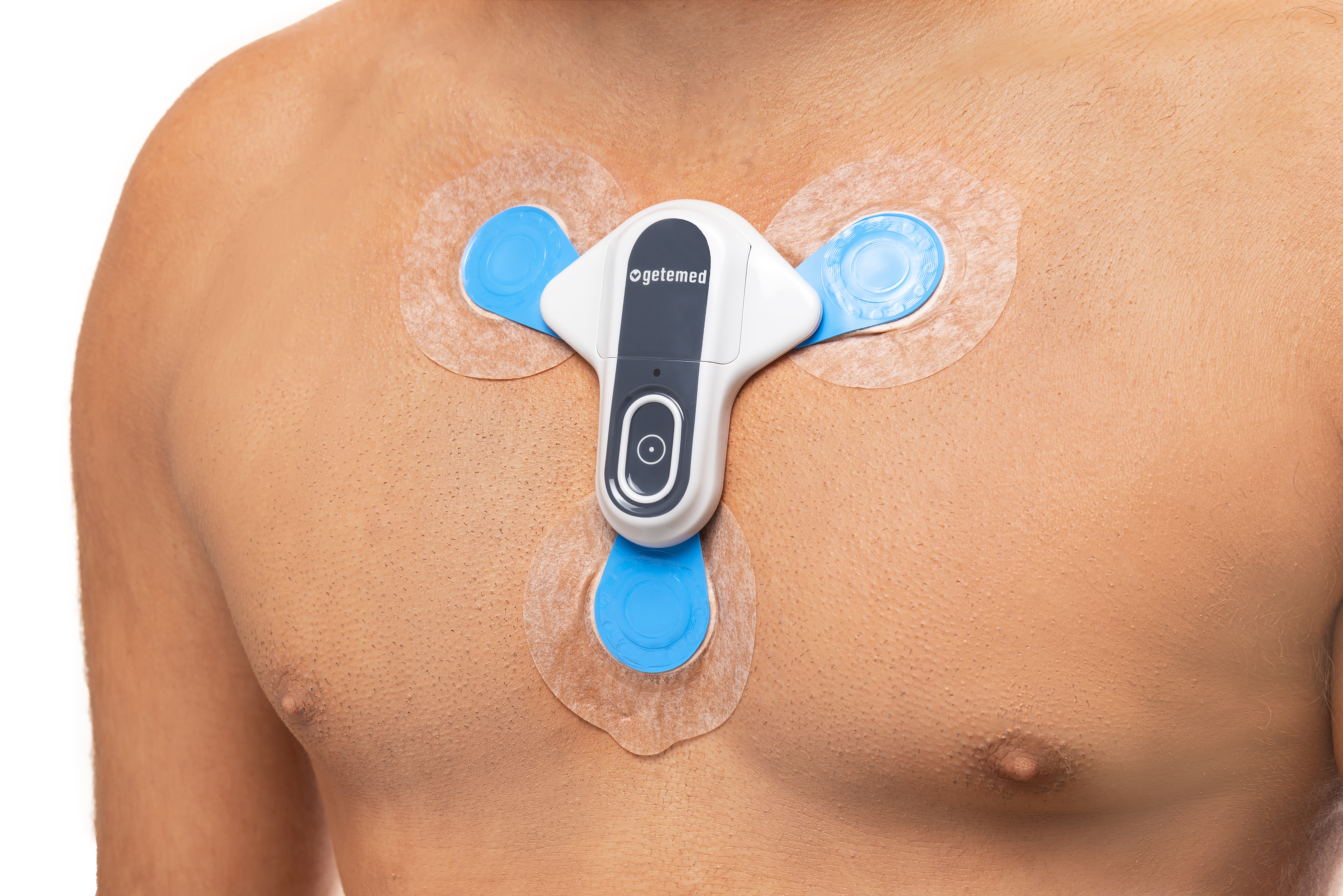
As the gold standard for diagnosing Pause, Afib and other arrhythmias is to elucidate the symptom-electrocardiograph (ECG) correlation. ECG monitoring is an established procedure in the evaluation and monitoring of patients with those abnormalities. The ECG recordings during those diseases allow physicians to confirm or exclude a Pause, Afib or other types of arrhythmias. Many studies have investigated the use of internal loop recorders (ILR), while there are recent studies of external loop recorders (ELR) for patients with unexplained syncope.
Unfortunately, ILRs carry the risk of pocket infections that resolve with device explanation. This complication, which can occur either in the peri-procedural phase or later during the follow-up, was reported in 1 to 5% of patients. Another disadvantage of ILR includes the presence of under-sensing or over-sensing.
And considering that the diagnostic yield of ELR was higher than standard 24h-Holter monitoring, it was similar to the diagnostic yield of ILR in the same timeframe. However, the limitations of ELR are patient compliance, failure to operate the device correctly, and difficulties handling cutaneous patch electrodes.
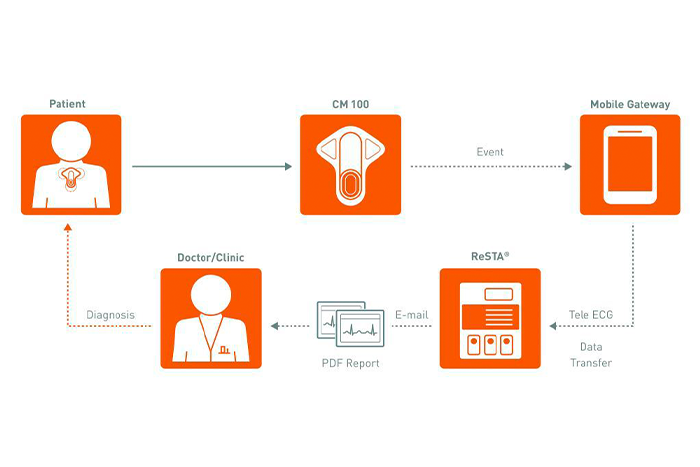
We believe that with the CM 100 technology we overcome all these limitations and, several cost-effectiveness analysis has indicated that the improvement in diagnostic yield offsets the cost of the ELR and that they are an economically attractive alternative when compared to Holter monitoring and the use of ELR could be viewed as a first-choice tool for patients with early recurrence of Pause, Afib and other Arrhythmias types and should be considered for patients for long-term ECG monitoring before ILR.



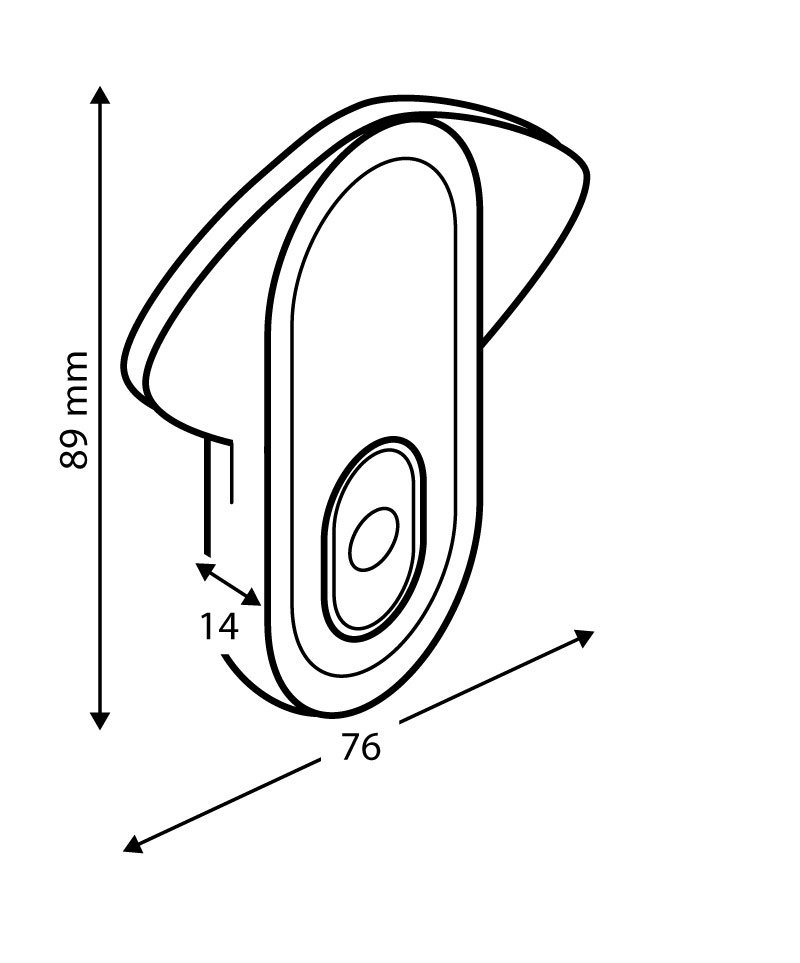

240422032331737~.jpg)